Explore Flucamp

August 3rd, 2023

July 2nd, 2023
Your clinical trial questions answered.
-
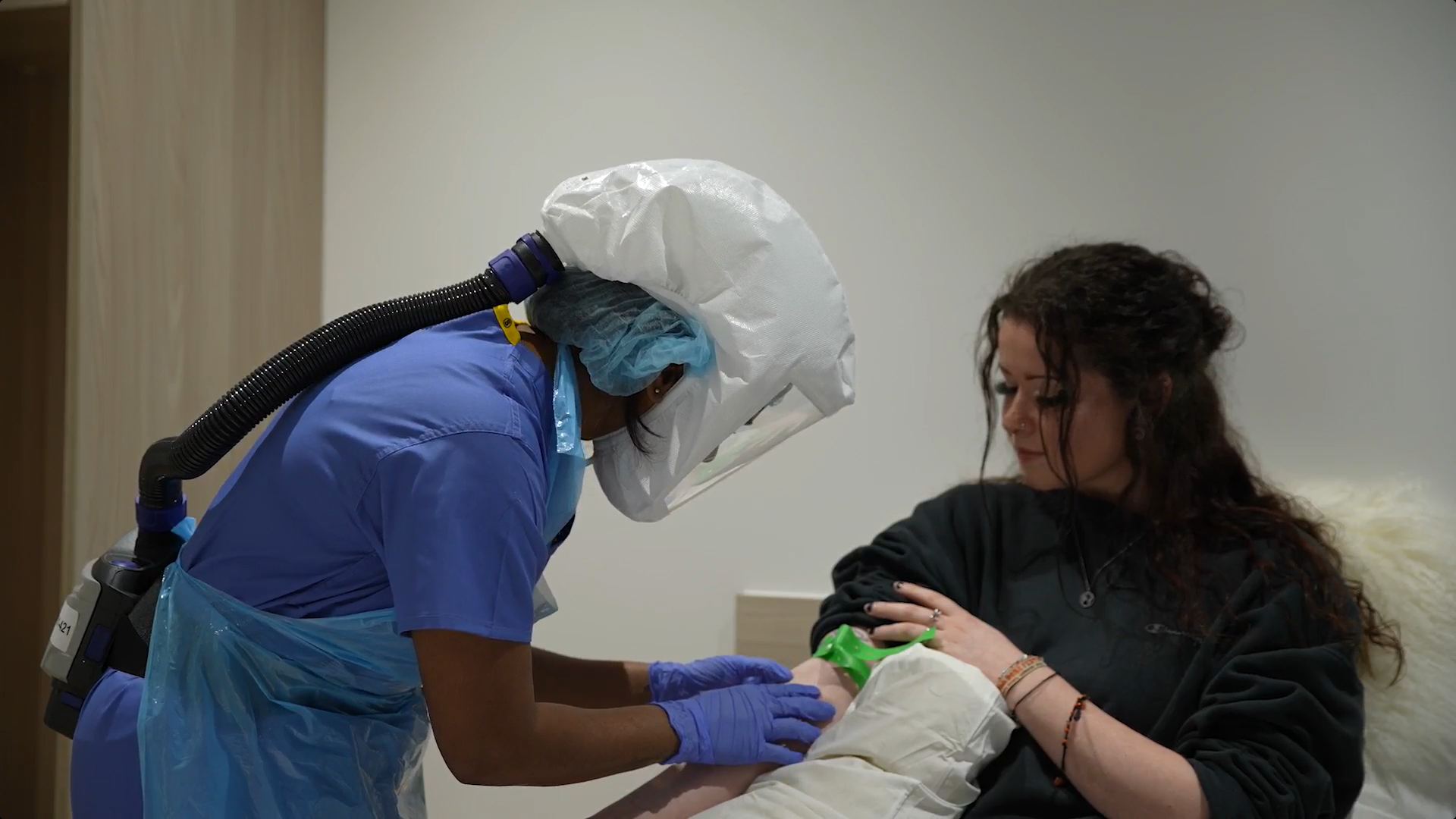
Volunteers joining FluCamp for a clinical trial stay with us on a residential basis in one of our purpose built rooms. Because it’s so important that the data we collect (from samples like blood and mucus ) is ‘clean’, volunteers must stay in quarantine conditions for the whole study. Additionally for this reason, our staff wear masks to comply with infection control guidelines. Please note, occasionally we may have studies available where no virus is administered and therefore residential may not be necessary.
-
Most of our clinical trials are residential, and are conducted under quarantine. This means that you will not be able to visit any relatives or friends who are taking part in a FluCamp clinical study during their stay. This is a strict rule as we need to ensure the safety of both our volunteers and others. The viruses we give are replications of those that are already out in the public domain, but nevertheless we would prefer not to spread them outside of FluCamp! Volunteers are allowed electronic devices such as laptops and smart phones so you are able to stay in touch with your family member/friend throughout their stay.
-
All of our work is conducted to the highest standards, and all medical trials that involve an investigational medicinal product are regulated by the Medicines and Healthcare Products Regulatory Agency. That means that every trial has been reviewed and approved by external bodies including the Research Ethics Committee as part of a quality management system. FluCamp operates a single quality management system to ensure compliance with relevant regulations and guidelines, applicable to working in the clinical trial industry and handling data. These include The Medicines for Human Use (Clinical Trials) Regulations 2004, Good Clinical Practice (GCP), Human Tissue Act 2004 and Data Protection Act 2018 – which are all regulated by external bodies. The performance and standards of our Clinical Teams are accountable to the same governing bodies as NHS staff e.g GMC for physicians. All of our trials are conducted in line with regulations and we take the utmost care of our volunteers. If you have any questions with regards to Flu Camp trial regulators then please get in touch.
-
We conduct research with respiratory viruses used in challenge studies, we are currently developing a model for research using SARs-COV-2.
-
You will be financially compensated for your participation in a clinical trial. The amount of compensation will be approved by an independent ethics committee and will be fully communicated in your informed consent form which will be provided prior to your participation in a clinical trial.